| Topic | Course | Course Materials | Readings |
|---|---|---|---|
| Introduction to Applied Pharmacokinetics | MPAS 650 | Slides Sample Questions Vancomycin Example | Ref. #1 |
| Renal Function Assessment | Slides Equations Cases | ||
| Therapeutic Drug Monitoring | PHAR:8370 IP Respiratory | Slides Equations Cases Sample TDM Questions | Ref. #1 |
| Vancomycin & Aminoglycoside CPK | Vancomycin Slides Aminoglycoside Slides Vancomycin Cases Aminoglycoside Cases CPK-Equations Additional Antibiotic Cases | Ref. #2 | |
| Clinical Pharmacokinetics and Hepatic Disease | PHAR:8372 IP GI and Nutrition | Slides Problem Set Cases | Ref. #3, Ref. #4 |
| Clinical Pharmacokinetics and Renal Disease | PHAR:8373 IP Renal and Electrolytes | Slides Renal Disease Problem Set |
Treatment of Minor Burns and Sunburns
ABA Burn Center Referral Criteria
American Burn Association Resources
Professional Discovery I students will develop skills in the scientific method, hypothesis testing, presentation of scientific knowledge in written and verbal form, and analysis of clinical study design.
According to the Centre for Evidence Based Medicine (CEBM), "one of the fundamental skills required for practicing EBM is the asking of well-built clinical questions. To benefit patients and clinicians, such questions need to be both directly relevant to patients' problems and phrased in ways that direct your research to relevant and precise answers."
A well-built clinical foreground question should have all four components. The PICO model is a helpful tool that assists you in organizing and focusing your foreground question into a searchable terms for a drug information query or dveloping a researchable topic. Here is the PICO model:
Evidence based medicine (EBM) has developed a levels of evidence pyramid to facilitate the understanding of the types of clinical questions and how they relate to study designs. The PICO model is a helpful tool that assists you in organizing and focusing your foreground question into a searchable terms for a drug information query or dveloping a researchable topic. Here is the PICO model:
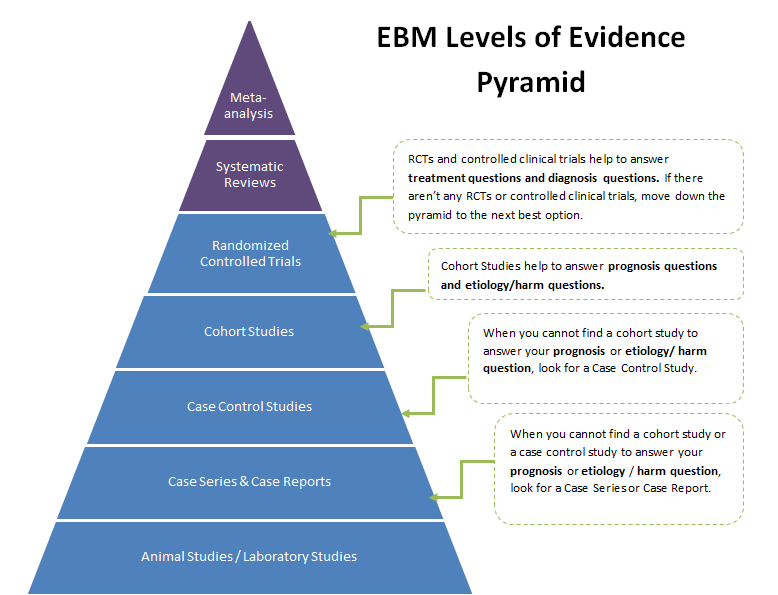
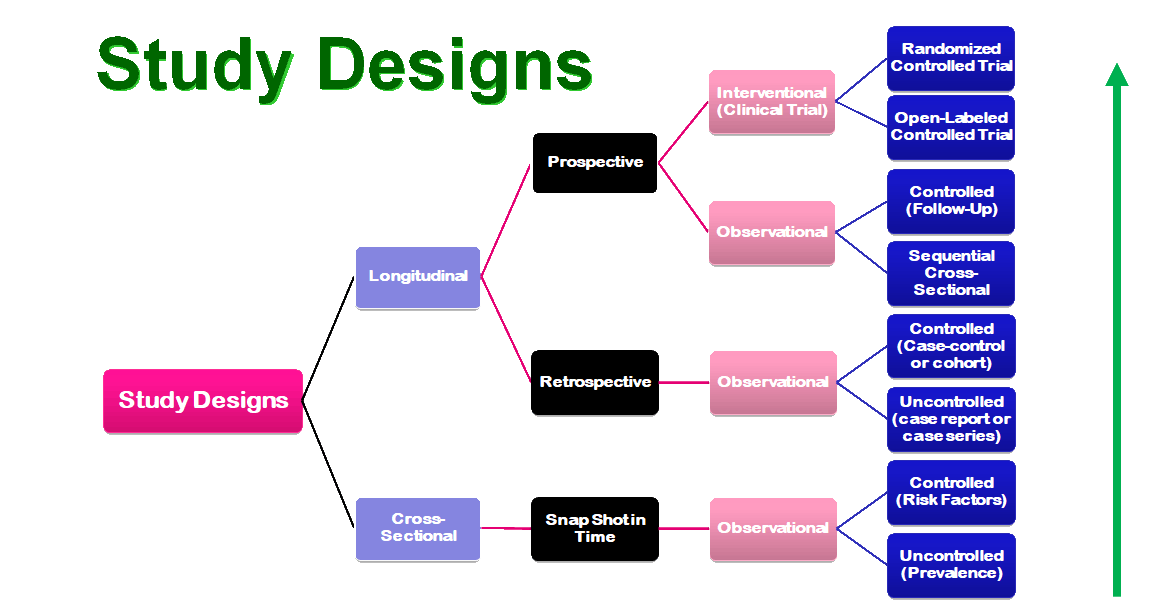
The University of Iowa Hospitals and Clinics is an 800+ bed tertiary care teaching hospital. The site is devoted to help students discover the skills and knowledge they need to become future pharmacy practitioners and researchers. A major focus of the experience is to expose students to the role of safe medical research through the review and approval of all proposed research that involves medications being used in humans. The Institutional Review Board (IRB) approve all research protocols and the preceptor will approve each of them from the Pharmacy & Therapeutics Committee perspective. Students and residents are exposed to the research process from the design and safety perspective and how these issues might impact pharmacy. A portion of the experience is also spent providing back-up coverage in the hospital drug information center.
Students who will practice in a hospital or a clinical practice environment will benefit from this rotation:
All rotation students must have a UIHC student ID badge in order to participate in rotations at the UIHC. If you have a UIHC student ID badge from a previous rotation at the UIHC, bring it with you. If you have never had an UIHC student ID badge, stop by the UIHC Pharmacy Administrative Offices (CC101GH, just south of the compass and Elevator B) to obtain the paperwork after 8:00 am on the first day of the rotation. Badging hours are Monday – Friday from 7:30 am to 5:00 pm.
APPE Students Only:
All students will need to complete Epic training prior to their first rotation at UIHC. Epic is our computerized medical record. Students will only need to complete this the first time that they are starting a rotation at UIHC. The training is done on-line by viewing a series of videos and then working in the Epic Playground site. The videos can only be viewed on UIHC computers so you will not be able to complete this prior to the start of the rotation. Computers are available either with your preceptor or in the student room at UIHC. Please bring earbuds or headphones as you may be on a computer in a room with other students watching the videos. Please contact Betsy Beltz (elizabeth-beltz@uiowa.edu) to get specific information for this training for your particular cycle.
PLEASE NOTE: YOU MUST COMPLETE THIS TRAINING EVEN IF YOU HAVE ATTENDED A PREVIOUS EPIC TRAINING SESSION FOR EMPLOYMENT REASONS OR RESEARCH PURPOSES.
Dr. Herman partners with Global Health Outreach which sends out about 50 medical teams to many parts of the world. They typical team has about 5 physicians, 5 dentists, 4 nurses, 2 pharmacists and several logistics personnel. Trips to Latin or South Americal are generally one week in length while trips to Africa and Asia are two weeks in length. The balance of the clerkship rotation is spent in pre-trip preparation and post-trip documentation and publication of the experience. The possible sites that can be arrange can be found on the Global Health Outreach website.
The student will spend time before and after the time at the underserved population at Dr. Herman’s normal place of practice, either at the College of Pharmacy or at the drug information center in the Department of Pharmaceutical Care at The University of Iowa Hospitals and Clinics. The location and length of time spent serving an underserved population will be negotiated with the group that the student and preceptor will participate with. Generally, Dr. Herman participates with Global Health Outreach (GHO). They send out about 50 medical teams a year that provide primary health care services in a variety of underserved areas. The teams (which include physicians, dentists, nurses, pharmacists and logistics personnel) that travel to Africa and Asia are generally 2 weeks in length and the teams that travel to Latin or South America are one week in length. The student and preceptor are each responsible for the costs associated with the trips. There are a variety of University and private sources that help cover the expenses. Generally the 2 week trips cost about $4,500 and the one week trips about $2500. That includes foreign and domestic travel, meals, accommodations, the processing of all government red-tape in the host country and the cost of medications that will be used on the trip. In addition, the University requires a $400 fee to process class credit and provide insurance for the student. Note that the student will need to apply for the trip and be accepted at least 2-3 months in advance of the scheduled trip.
P4 standing. Also the student will need to work with the preceptor well in advance to select the trip, apply for the trip according to the partnering organizations procedures, agree to the code of conduct of the partnering organization, agree to the fees associated with the trip, and agree to fully participate with the trip scheduled activities.